The titles of each poster/paper will link out to ResearchGate for the full version download
Updated January 26, 2022
2022
Cutaneous pharmacokinetics of escalating Ruxolitinib formulation doses utilizing a coherent Raman imaging pipeline
Visualizing and Quantifying Drug Distribution in Tissue VI
Systemic drug delivery for dermatological conditions yields little drug to the intended site of action resulting in adverse effects. Topical drug delivery is a viable alternative yet the local cutaneous pharmacokinetics (cPK) is relatively under-explored. Product dosing is dependent upon the knowledge of the dose-cPK relationship, which coincides with the pharmacodynamic (PD) activity. Coherent Raman imaging (CRI) can quantify tissue-specific drug localization and elucidate micro-scale cPK estimates, affording a clinically relevant cPK-PD relationship. This demonstration of a dose-cPK relationship utilizing CRI offers a stepping stone for additional formulation evaluation ex vivo.
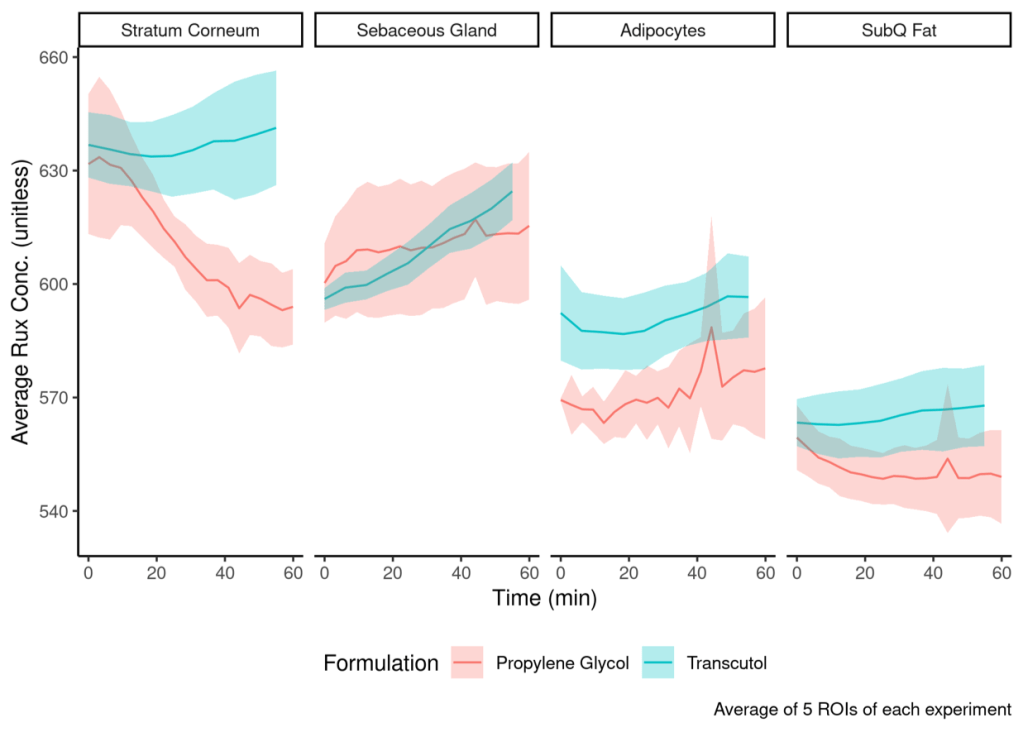
The earlier the better: instantaneous cutaneous concentration quantification at the time of application
Visualizing and Quantifying Drug Distribution in Tissue VI
Cutaneous pharmacokinetics (cPK) can be quantified utilizing a myriad of in vitro and in vivo approaches. However, these techniques provide macroscale cPK for long experimental durations and do not provide cPK information from the moments directly after a topical formulation application, thus missing critical product dosing insight. Furthermore, formulations are typically applied for 5-10 minutes in the clinic and then removed (purposely or accidentally), which requires a high temporal and spatial resolution estimate of the early timepoint cPK. We have developed a 3-D printed applicator to address the unmet need for early time-point cPK quantification pertinent to the clinic.
Patent Pending for this device
2020
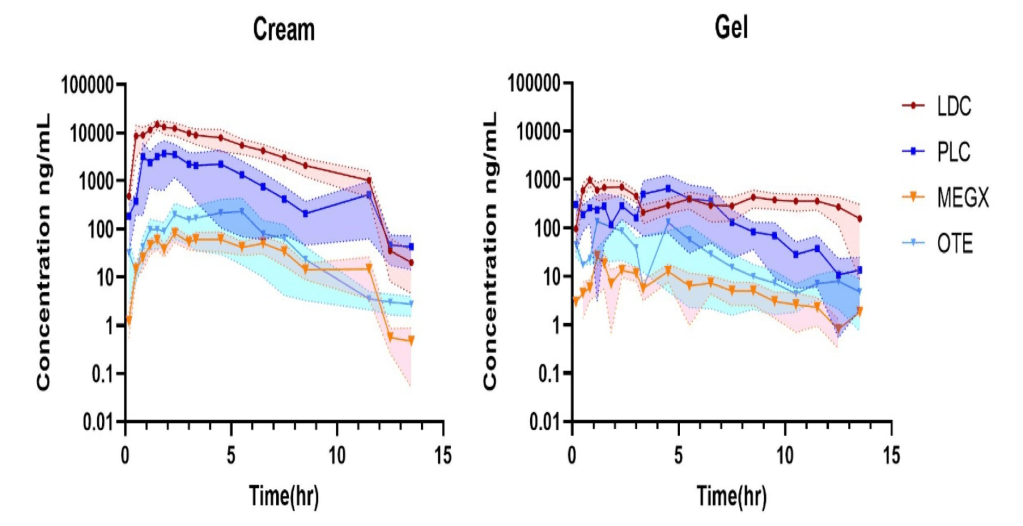
Dermal Pharmacokinetics of Lidocaine, Prilocaine and their Metabolites from Topical Dermatological Drug Products
AAPS PharmSci 360 – 2020
The research presented in this poster focuses on the evaluation of the capability of dermal microdialysis (dMD) to assess the dermis bioavailability of LDC/PLC TDDPs and their corresponding dermal metabolites. Although most of the major drug-metabolizing enzymes are expressed in the skin very little is known about cutaneous metabolite formation and their pharmacokinetics. Skin metabolism is important when considering drug discovery and safety assessment and the efficient use of topical dermatological products. However, limitations in sampling techniques and analytical capabilities deemed the characterization of skin metabolites impossible, up to date. Dermal microdialysis is an ultrathin, semipermeable hollow fiber which is placed in the dermis and continuously samples the dermal interstitial fluid and permits to quantify soluble and unbound compounds that are present in the extracellular fluid of the dermis, which are also responsible for therapeutic effect in the skin. Therefore, when dMD technique is coupled with an extremely sensitive analytical technique like LC-MS/MS, also metabolites can be quantified.
2019
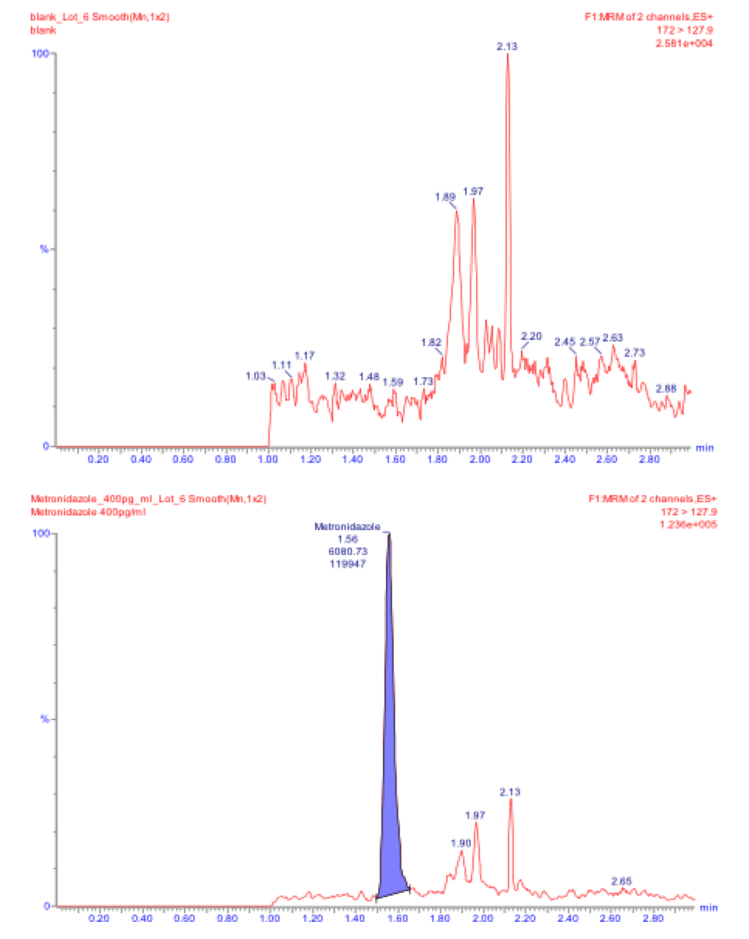
Evaluating the Bioequivalence of Topical Dermatological Drug Products containing Metronidazole using Dermal Microdialysis: Preliminary Studies in Rabbits
AAPS PharmSci 360 2019
The availability of high-quality, generic drug products can substantially reduce the cost of health care. While plasma drug concentration data can be used to evaluate the bioequivalence (BE) of systemically acting drug products, comparative clinical-endpoint studies were historically used to evaluate the BE of topical dermatological drug products, which act locally in the skin or surrounding tissues. The 24-hour sampling duration appeared to be sufficient to characterize the complete dermal PK profile for MTZ in rabbit skin. Reference gel vs. cream products were accurately and sensitively discriminated as not being bioequivalent.
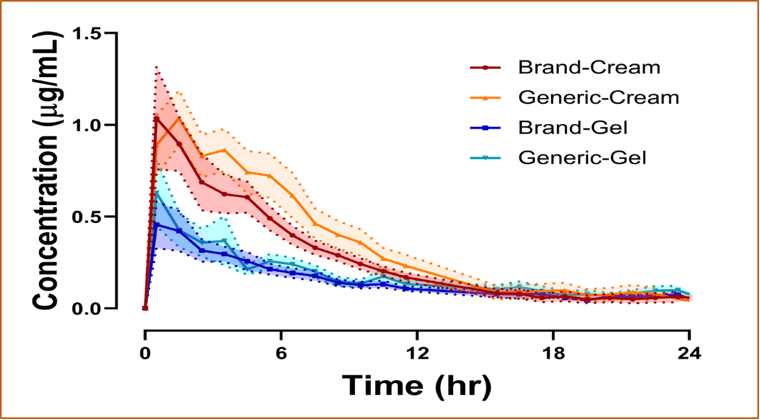
Estimation of In Vivo Percutaneous Permeation (Flux) and Cumulative Amount Input of Metronidazole Formulations in Mini-Pigs’ Dermis
AAPS PharmSci 360 2019
A dermal microdialysis (dMD) probe placed within the dermis below a topically applied formulation can measure the changes in drug concentrations in the dermis over time; however, this does not distinguish absorption from distribution and elimination occurring in the dermis.
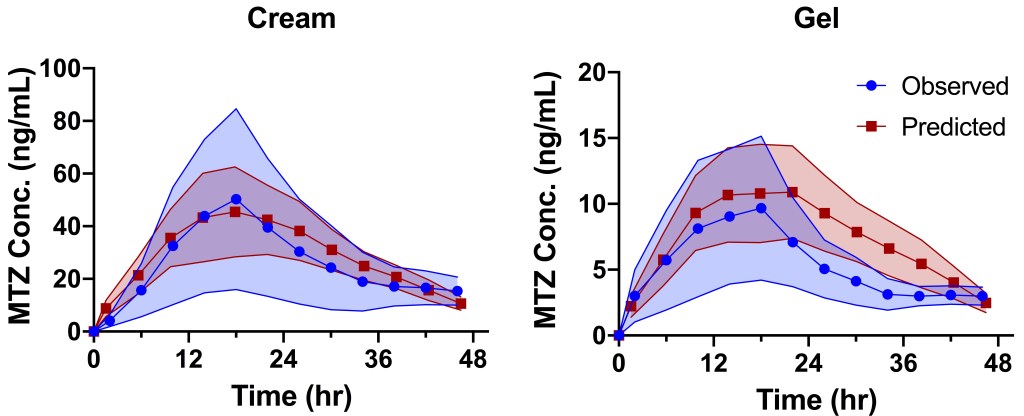
2018
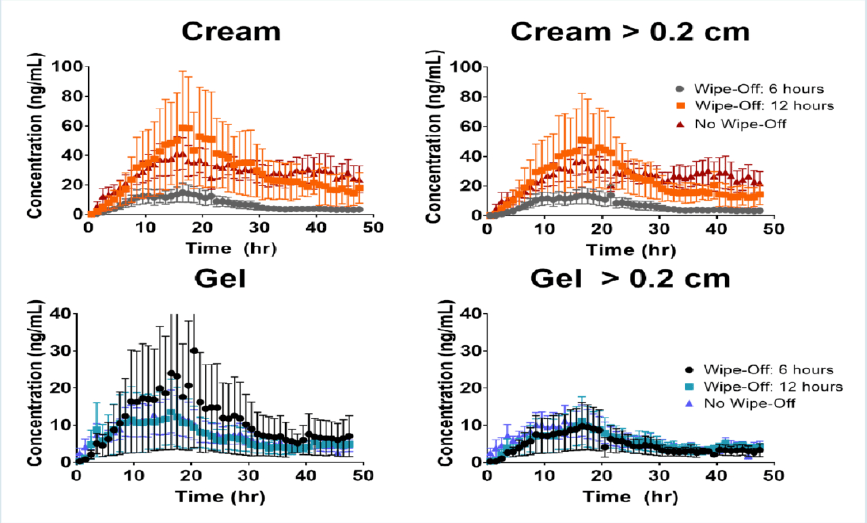
Effect of formulation wipe-off time on topical bioavailability of metronidazole using dermal microdialysis
AAPS PharmSci 360 – 2018
The in-vivo rate of absorption from a traditional topical drug formulation (a cream or gel) may be a very slow process according to the experiments conducted with intradermal samplings, such as microdialysis or microperfusion. Continuous monitoring even for up to 48 hours may still result in a poor characterization of the elimination phase from the dermis. The study of formulation wipe-off effect on the dermis pharmacokinetics of topical formulations would permit a complete characterization of both the absorption and elimination phases in a reasonable time frame. The goal of the study was to assess the effect of formulation removal on dermis exposure resulting from the application of 10 mg/cm2 of two metronidazole (MTZ) topical dermatological formulations compared to non-removal. The difference between terminal half-life at formulation sites and formulation-independent elimination t½ suggests a flip/flop scenario. The results of these studies further confirm the capability of the microdialysis technique to capture differences in the rate and extent of dermis absorption from topical dermatological formulations.
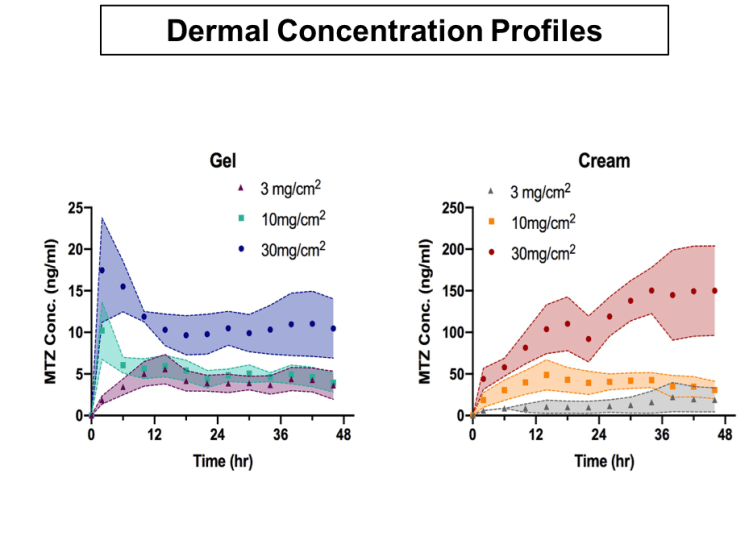
Evaluation of the local bioavailability from topical formulations using dermal microdialysis
AAPS PharmSci 360 – 2018
Dermal microdialysis (dMD) can directly monitor the rate and extent to which a topically administered drug becomes available in the dermis, at or near the site of action in the skin. In dMD, microdialysis probes traverse the dermis just beneath the skin surface, spanning the area of a test site. Using multiple test sites on the same subject, and replicate probes at each test site, it is feasible to compare the cutaneous pharmacokinetics (PK) of a drug from different products in parallel on the same subject.
LC-MS/MS Method for the Quantification of Metronidazole in skin dialysate
AAPS PharmSci 360 – 2018
Intradermal microdialysis (dMD) facilitates the monitoring of drug concentration in the dermis – the site of action for many topically applied dermatological products. The success of the technique is strictly connected with the availability of a highly sensitive and selective analytical method. A UPLC-MS/MS for the quantification of Metronidazole (MTZ) and D3-Metronidazole (D3-MTZ) was developed and validated. The method was successfully applied to pharmacokinetic studies of metronidazole dermis dialysate using microdialysis